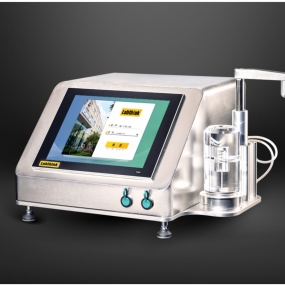
C840H Integrated Evaporation Residue Testing System
-
888
-
0765 259 545
C840H Integrated Evaporation Residue Testing System
Model: C840H
Labthink - China
C840H Integrated Evaporation Residue Testing System is designed and produced based on the principle of gravimetric method measurement and testing standards for plastic packaging and chemical reagents, etc. It is suitable for the determinations of evaporation residue of food or pharmaceutical packaging, total migration of food contact or pharmaceutical contact materials or products, and evaporation residue of chemical reagents and purified water.
Model: C840H
Labthink - China
C840H Integrated Evaporation Residue Testing System is designed and produced based on the principle of gravimetric method measurement and testing standards for plastic packaging and chemical reagents, etc. It is suitable for the determinations of evaporation residue of food or pharmaceutical packaging, total migration of food contact or pharmaceutical contact materials or products, and evaporation residue of chemical reagents and purified water.
C840H Integrated Evaporation Residue Testing System
Model: C840H
Labthink - China
Model: C840H
Labthink - China
Test Standard Compliance:
ISO 1135-4:2015, ISO 1135-5:2015, ISO 3826-1:2019, ISO 3826-4:2015, ISO 8536-4:2019, Pharmacopoeia, YBB00342002-2015, YBB00132002-2015 and other standards for pharmaceutical production and pharmaceutical packaging.
EN1186-3, GB 31604.8-2016, GB/T 5009.60 and other standards for food contact materials.
ISO 759-1981, ISO 6353-1:1982 GM14, GB/T 9740 and other related standards for determination of chemical reagent residue after evaporation.
EN1186-3, GB 31604.8-2016, GB/T 5009.60 and other standards for food contact materials.
ISO 759-1981, ISO 6353-1:1982 GM14, GB/T 9740 and other related standards for determination of chemical reagent residue after evaporation.
Applications:
|
Basic Applications
|
Purified Water
|
Determination of non-volatile matters in purified water for pharmaceutical applications.
|
|
Extensive Applications
|
Pharmaceutical Packaging Materials
|
Determination of non-volatile matters of various pharmaceutical composite films, bags, bottles, rubber plugs and caps.
|
|
Food Contact Materials
|
Determination of the total migration amount of polyethylene, polystyrene, polyvinyl chloride, polypropylene, melamine, foam polystyrene and plant fiber molding products.
|
|
|
Chemical Reagents
|
Determination of various chemical reagent residues after evaporation.
|
Technical Parameters
Table 1: Test Parameters Note 2
|
Parameter\Model
|
C840H
|
|
|---|---|---|
|
Testing Range
|
mg
|
0.05~10000
|
|
0.3~80000 (optional)
|
||
|
Resolution
|
mg
|
0.01
|
|
0.1 (optional)
|
||
|
Repeatability
|
mg
|
±0.05
|
|
±0.3 (optional)
|
||
|
Temperature Range
|
℃
|
Room temperature~130
|
|
Temperature Fluctuation
|
℃
|
±0.5
|
|
Extended Functions
|
21 CFR Part11
|
optional
|
|
2Computer system requirements for GMP
|
optional
|
|
Table 2: Technical Specifications
|
Test Stations
|
25
|
|
Test Cup Volume
|
100mLNote 3
|
|
Gas Specifications
|
Compressed air (gas source is provided by the user)
|
|
Gas Source Pressure
|
≥ 72.5 PSI/500 kPa
|
|
Port Size
|
Φ8mm Polyurethane tube
|
|
Instrument Mainframe Dimensions
|
32.6” H x 43.3” W x 28.7” D (83cm × 110cm × 73cm)
|
|
Power Supply
|
120VAC±10% 60Hz / 220VAC±10% 50Hz (Select one from the two)
|
|
Net Weight
|
440Lbs (200kg)
|
Product Similar
C403H Oxygen /Water Vapor Transmission Rate Test System
Model: C403H
Labthink - China
C403H Oxygen /Water Vapor Transmission Rate Test System is based on the testing principle of Coulometric oxygen sensor and infrared water vapor sensor. It is designed and manufactured according to ASTM D3985、ASTM F1249,ISO 15106-2 and other relevant standards to provide high precision and high efficiency oxygen and water vapor transmission rate tests for high and medium gas barrier materials. It is suitable for testing the oxygen and water vapor transmission performance of films, sheets and related materials in the fields of food, medicine, medical devices, daily chemicals, photovoltaic, electronic and many others.
0765 259 545
C690M Nondestructive Package Leakage Detector
Model : C690M
Labthink - China
C690M Nondestructive Package Leakage Detector is based on the vacuum decay method and pressure decay method, and is designed and manufactured according to ASTM F2338 and other standards. It is professionally suitable for the trace leakage detection of various drug packaging such as vials, ampoule bottles, cartridge bottles, infusion bottle, and prefilled syringes and so on.
0765 259 545
BTY-B3P Gas Permeability Tester
Model: BTY-B3P
BTY-B3P Gas Permeability Tester is based on the differential pressure method, and is professionally applicable to the determination of gas permeability of battery diaphragms, breathable films and other relative polymer products.
0765 259 545
C180M Air Permeability Tester
Model: C180M
Manufacturer : Labthink - China
C180M Air Permeability Tester is based on the principle of differential pressure method and is professionally suitable for air permeability tests of cigarette papers, filter plug wrap, filter joining paper and materials with an oriented permeable zone.
Reference Standards
ISO 2965, YC/T172, GB/T 12655, and JJG (Tobacco) -1998
0765 259 545
- Digital P.I.D. temperature control technology ensures the preset temperature to be reached rapidly without any fluctuation
- The instrument is heated through fluid medium, which could provide the stable test environment
- Automatic timer function to efficiently guarantee the accuracy of test data
- The instrument is controlled by micro-computer with LCD, PVC operation panel, and menu interface
- Standard clamp wire mesh support could make the test done smoothly
GB/T 13519, ASTM D2732
0765 259 545
FIT-01 Film Pendulum Impact Tester
Model: FIT-01
FIT-01 Film Pendulum Impact Tester is professionally applicable to the determination of impact resistance properties of pendulum of plastic films, sheets, composite films, aluminum foils and other materials.




 SCITEK - China
SCITEK - China EBP - China
EBP - China SOOHOW - China
SOOHOW - China ZD Instrument - China
ZD Instrument - China Yante
Yante JJ-Test
JJ-Test Labthink
Labthink Hunterlab
Hunterlab EURPING - China
EURPING - China Wisdom - China
Wisdom - China ZYLAB - CHINA
ZYLAB - CHINA ERKAYA -
ERKAYA - Novotest - Ukraine
Novotest - Ukraine Moderner - China
Moderner - China GBPI - China
GBPI - China Milkotester - Bulgaria
Milkotester - Bulgaria LAMY RHEOLOGY
LAMY RHEOLOGY  Emco
Emco GESTER - China
GESTER - China GonDo - Taiwan
GonDo - Taiwan Agri-instrument - China
Agri-instrument - China CHN - China
CHN - China PNTOO - China
PNTOO - China TESTER SANGYO
TESTER SANGYO FRU - China
FRU - China Rucca - China
Rucca - China HSIANG TAI - CHINA
HSIANG TAI - CHINA FYI - China
FYI - China Boxun - China
Boxun - China Linshang - China
Linshang - China Hanon - China
Hanon - China PCE - UK
PCE - UK  Biuged
Biuged IRIS
IRIS ACEY - China
ACEY - China 3NH
3NH XS Instruments
XS Instruments Doser
Doser OPTIKA - ITALY
OPTIKA - ITALY Great Safe
Great Safe Kett
Kett Mitutoyo
Mitutoyo Cometech - Taiwan
Cometech - Taiwan Veego
Veego COPLEY SCIENTIFIC
COPLEY SCIENTIFIC  Exotek
Exotek Total Meter
Total Meter PNShar
PNShar Radwag
Radwag SH Scientific
SH Scientific X-Rite
X-Rite Metrotec
Metrotec Hach
Hach Hanna
Hanna Endecotts
Endecotts TRINAMIX
TRINAMIX HMKTEST
HMKTEST PackTest Machines Inc
PackTest Machines Inc CHAO QIANG - CHINA
CHAO QIANG - CHINA Sartorius - Germany
Sartorius - Germany SIKA - Germany
SIKA - Germany Ohaus
Ohaus SUN SCIENTIFIC
SUN SCIENTIFIC Aczet
Aczet Trace2o - UK
Trace2o - UK Glas -Col - USA
Glas -Col - USA Beijing
Beijing ATAGO – JAPAN
ATAGO – JAPAN TECHLAB SYSTEMS
TECHLAB SYSTEMS ESI
ESI RJS - USA
RJS - USA Kruss
Kruss Lumex Instruments
Lumex Instruments ELECTROLAB
ELECTROLAB  Haida
Haida Horiba
Horiba Konica
Konica Datacolor
Datacolor CRYSTE
CRYSTE Uni-T
Uni-T Oxford Instruments
Oxford Instruments Fuzhou Furi - China
Fuzhou Furi - China Alpha MOS
Alpha MOS  Drick
Drick FPInnovations - Canada
FPInnovations - Canada Memmert
Memmert 3M
3M Advantec
Advantec Newstar
Newstar ANTON PAAR
ANTON PAAR